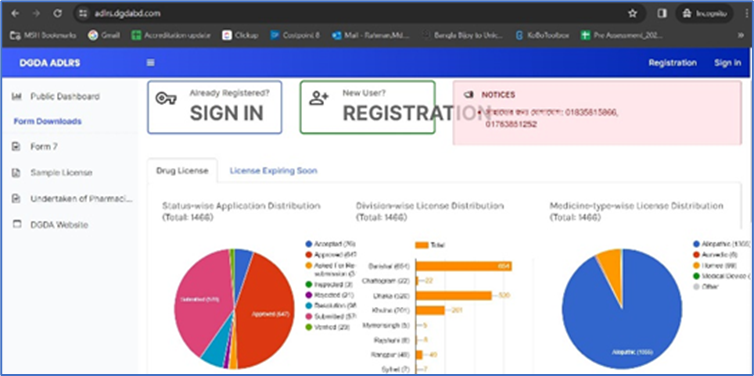
Through a recent Office Order issued by DGDA on 31 October 2023, the regulatory authority mandated that the country’s retail medicine outlets must submit applications for new and renewal drug licenses online through ADLRS (Automated Drug Licensing and Renewal System) rather than through the manual system.

DGDA officials will also process the applications and documents online using the ADLRS software, which BHB helped DGDA to develop. Recently, BHB made a significant upgrade to the software with the continued financial assistance from the UK Foreign, Commonwealth and Development Office. Medicine outlets can access the software at this url: https://adlrs.dgdabd.com/